electron configuration lithium|where does lithium come from : Bacolod Electron configurationThe arrangements of electrons above the last (closed shell) noble gas. Melting pointThe temperature at which the solid–liquid phase change occurs. . ดาวน์โหลด AirAsia MOVE วันนี้แล้วรับข้อเสนอที่ดีที่สุดทั้งเที่ยวบิน โรงแรม บริการรับส่ง และอื่นๆ อีกมากมาย! จบทริปครบจบในแอปเดียว.
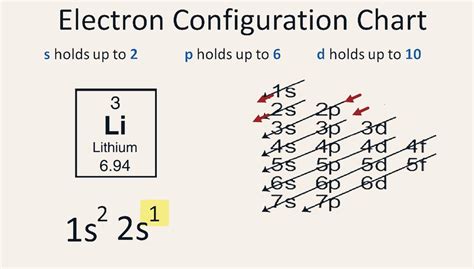
electron configuration lithium,Mar 23, 2023 electron configuration lithium where does lithium come fromClick on above elements (in Periodic table) to see their information or Visit .How to Write the Electron Configuration for Lithium. Lithium is the third element with a total of 3 electrons. In writing the electron configuration for lithium the first two . Lithium Electron Configuration. A step-by-step description of how to write the electron configuration for Lithium (Li). In order to write the Li electron configuration we first need.Electron configurationThe arrangements of electrons above the last (closed shell) noble gas. Melting pointThe temperature at which the solid–liquid phase change occurs. . 759K subscribers. 48K views 4 years ago. .more. In this video we will write the electron configuration for Li+, the Lithium ion. We’ll also look at why Lithium forms a 1+ ion and .Electron Configuration of Sodium and Lithium; Sodium 1s 2 2s 2 2p 6 3s 1 = [Ne]3s 1; Lithium: 1s 2 2s 1 = [He]2s 1
where does lithium come from Two of the lithium electrons can fit into the 1s subshell, but the third electron must go into the second shell and the lower energy orbital, which is the 2s . Lithium is a rare element found primarily in molten rock and saltwater in very small amounts. It is understood to be non-vital in human biological processes, . Learn how to write electron configurations for atoms, including lithium, using the aufbau principle and subshells. Watch a video, see examples, and read . Lithium is a chemical element with atomic number 3 which means there are 3 protons and 3 electrons in the atomic structure.The chemical symbol for Lithium is Li. It is a soft, silvery-white alkali metal. Under standard conditions, it is the lightest metal and the lightest solid element. Like all alkali metals, lithium is highly reactive and flammable, . A step-by-step description of how to write the electron configuration for Lithium (Li). In order to write the Li electron configuration we first need to kno.
Lithium is an alkali metal with the atomic number = 3 and an atomic mass of 6.941 g/mol. This means that lithium has 3 protons, 3 electrons and 4 neutrons (6.941 - 3 = ~4). Being an alkali metal, lithium is a soft, flammable, and highly reactive metal that tends to form hydroxides. It also has a pretty low density and under standard conditions . Electron configuration chart of all Elements is mentioned in the table below.The Shorthand electron configuration (or Noble gas configuration) as well as Full . Electron configuration of Lithium (Li) [He] 2s 1: 1s 2 2s 1: 2, 1: 4: Electron configuration of Beryllium (Be) [He] 2s 2: 1s 2 2s 2: 2, 2: 5: Electron configuration of .

La configuration électronique du lithium est [He] 2s1. . Tous les éléments sont identifiés par leur numéro atomique Z, qui est le même que le nombre de protons et d'électrons dans leur noyau. Dans le cas du lithium, il correspond au numéro atomique 3. Dans ce cas, l'atome de lithium a 3 électrons, deux d'entre eux remplissent l .For hydrogen, therefore, the single electron is placed in the 1s orbital, and the electron configuration (also known a spdf notation) . Using this notation to compare the electron configurations of sodium and lithium, we have: Sodium: 1s 2 2s 2 2p 6 3s 1 = [Ne]3s 1: Lithium: 1s 2 2s 1 = [He]2s 1:For instance, lithium (Li ) has three electrons: two fill the 1 s orbital, and the third is placed in the 2 s orbital, giving an electron configuration of 1 s 2 2 s 1 . Neon ( Ne ), on the other hand, has a total of ten electrons: two are in its innermost 1 s orbital and eight fill the second shell—two each in the .
Its electron configuration is. He: 1s2 He: 1 s 2. The three electrons for Li are arranged in the 1s subshell (two electrons) and the 2s subshell (one electron). The electron configuration of Li is. Li: 1s22s1 Li: 1 s 2 2 s 1. Be has four electrons, two in the 1s subshell and two in the 2s subshell.Because lithium’s final electron goes into the 2s subshell, we write the electron configuration of a lithium atom as 1s 2 2s 1. The shell diagram for a lithium atom is shown below. The shell closest to the nucleus (first shell) has 2 dots representing the 2 electrons in 1 s , while the outermost shell ( 2 s ) has 1 electron.

This means that a neutral lithium atom will have a total of 3 electrons surrounding its nucleus. Its electron configuration will be. Li: 1s22s1. Now, the lithium cation, Li+, is formed when lithium loses the electron located on its outermost shell → its valence electron. This electron is located on the second energy level, in the 2s-orbital.The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. We describe an electron configuration with a symbol that contains three pieces of information ( .electron configuration lithiumSarah Faizi (University of California Davis) 2.4 Electron Configurations is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. The electron configuration of an atom is . Two of the lithium electrons can fit into the 1s subshell, but the third electron must go into the second shell and the lower energy orbital, which is the 2s orbital. Therefore, we write the electron configuration of a lithium atom as 1s 2 2s 1 (spoken as “one-ess-two two-ess-one”). The shell diagram for a lithium atom (Figure \(\PageIndex .The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. In the periodic table, the elements are listed in order of increasing atomic number Z. Electron configuration of Lithium is [He] 2s1. Possible oxidation states are +1.
Two of the lithium electrons can fit into the 1s subshell, but the third electron must go into the second shell and the lower energy orbital, which is the 2s orbital. Therefore, we write the electron configuration of a lithium atom as 1s 2 2s 1 (spoken as “one-ess-two two-ess-one”). The shell diagram for a lithium atom (Figure \(\PageIndex .Electron Configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table. The electron configuration for the first 10 elements. H .Two of the lithium electrons can fit into the 1s subshell, but the third electron must go into the second shell and the lower energy orbital, which is the 2s orbital. Therefore, we write the electron configuration of a lithium atom as 1s 2 2s 1 (spoken as “one-ess-two two-ess-one”). The shell diagram for a lithium atom (Figure \(\PageIndex .For example, the electron configurations of the first four elements, hydrogen, helium, lithium, and beryllium, look like. 1 s 1 1 s 2 1 s 2 2 s 1 1 s 2 2 s 2 and so on for the remaining elements. When chemists write down the really long electron configuration of an atom with a large atomic number (and thus lots of electrons .
electron configuration lithium|where does lithium come from
PH0 · where does lithium come from
PH1 · lithium electron configuration excited state
PH2 · list of electron first stages
PH3 · how to find electron configuration
PH4 · electron configuration for every element
PH5 · electron configuration explained
PH6 · electron configuration chart
PH7 · electron configuration calculator
PH8 · Iba pa